General Formula of Alkane
Hence the 2C 2 in the formula is the total number of hydrogen atoms that should be bound to the saturated carbon atoms and H is the number of hydrogen atoms that are actually present in the compound. By joining the carbon atoms in a ring you have had to lose two hydrogen atoms.
Alkane Molecular Structural General Formula Youtube
Pentan-1-yl is an example of a name by this method and is synonymous with pentyl from the previous guideline.

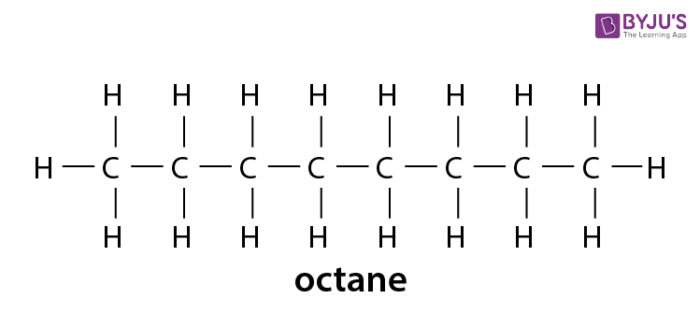
. Identify and name groups attached to this chain. In organic chemistry an alkane or paraffin a historical trivial name that also has other meanings is an acyclic saturated hydrocarbonIn other words an alkane consists of hydrogen and carbon atoms arranged in a tree structure in which all the carboncarbon bonds are single. Some types of oils and waxes are the examples of alkanes with many carbon atoms number.
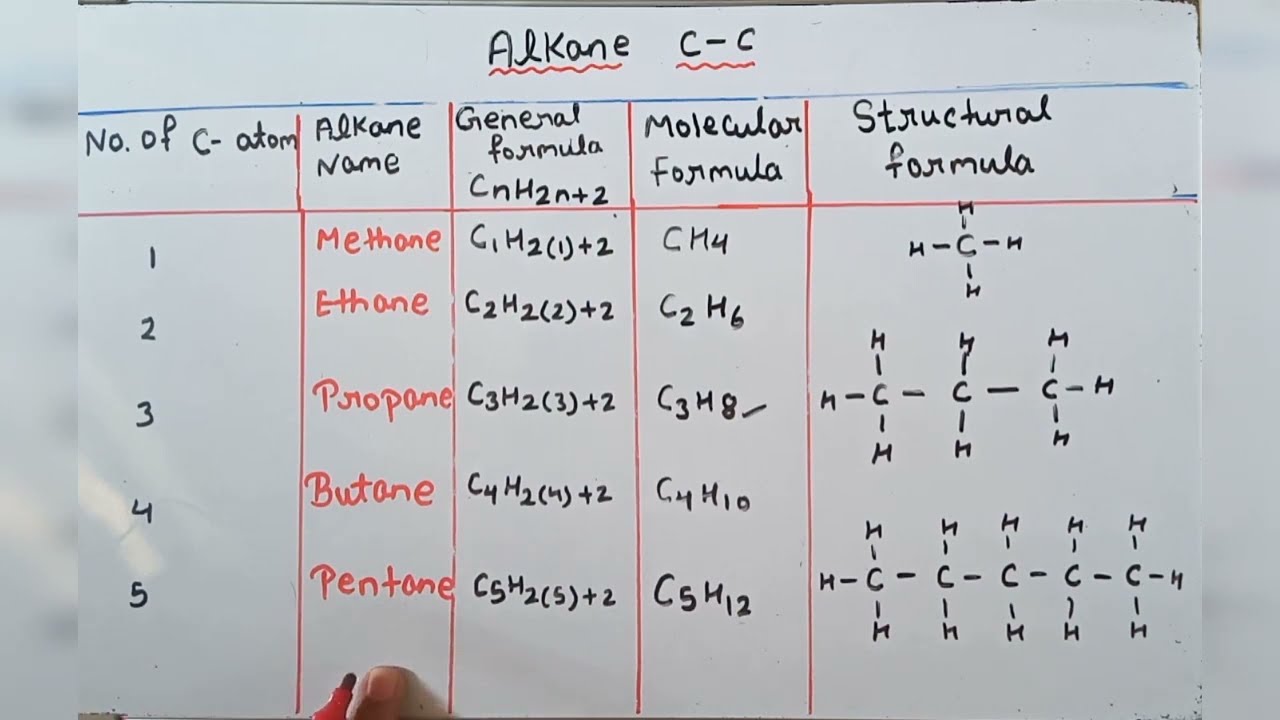
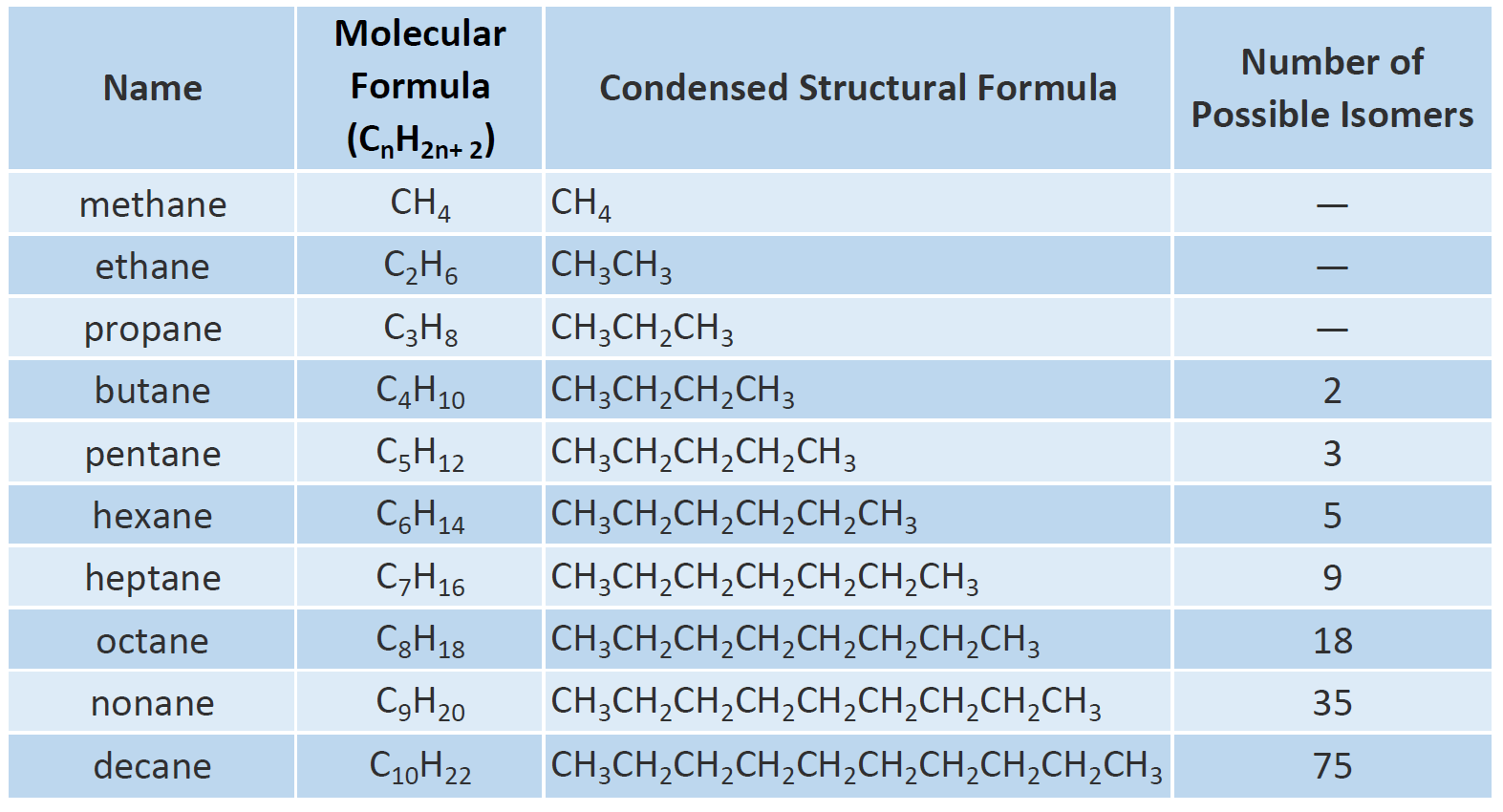
The simplest alkane is methane with the formula ofCH4. Find and name the longest continuous carbon chain. Problem 132 Write structur es of dif fer ent isomeric alkyl gr oups corr esponding to the molecular for mula.
They have the general formula C n H 2nWhere n is said to be the number of carbon atoms present in the organic compound. It consists of four hydrogen atoms and one carbon atom and is the simplest alkane. The double bond between carbon and oxygen is characteristic of all aldehydes and is known as the.
General Structure or Formula. This ring is formed due to their saturated nature and they have three compounds of alkane present in the structure which helps them in forming a ring. Read complete General Organic Chemistry notes for Class 11 Chemistry.
Cycloalkanes are the class of hydrocarbons having a ring-like structure. Paraffin hydrocarbon also called alkane any of the saturated hydrocarbons having the general formula CnH2n2 C being a carbon atom H a hydrogen atom and n an integer. If you count the carbons and hydrogens you will see that they no longer fit the general formula C n H 2n2.
Alkanes have the general chemical formula C n H 2n2The alkanes range in complexity from. The paraffins are major constituents of natural gas and petroleum. It also can be more than 10 carbon atoms.
Paraffins containing fewer than 5 carbon atoms per molecule are usually gaseous at room temperature those having 5 to 15 carbon atoms are. Let us recall the general rules for nomenclature already discussed in Unit 12. The general formula for alkanes is CnH2n 2.
General formula for alkyl groups is CnH2n1 Unit 12. You can also read. Methane is the simplest of saturated hydrocarbons with a chemical formula CH 4.
In other words a saturated form of a hydrocarbon will have the maximum number of hydrogen atoms in an acyclic alkane form. These notes covers the complete syllabus of General Organic Chemistry Class 11 including competitive exams like JEE mains and advanced NEET and others. IUPAC Rules for Alkane Nomenclature.
Aldehyde any of a class of organic compounds in which a carbon atom shares a double bond with an oxygen atom a single bond with a hydrogen atom and a single bond with another atom or group of atoms designated R in general chemical formulas and structure diagrams. There is no limit of how much carbons can be tied together. Nomenclature of substituted alkanes can further be understood by considering the following problem.
These are very detailed and comprehensive notes developed by team of expert faculties. When natural methane reaches the surface of the atmosphere is called atmospheric methane and can be found under the seafloor as well as below the ground. The more general method omits only the terminal e of the substituent name but requires explicit numbering of each yl prefix even at position 1 except for -ylidyne which as a triple bond must terminate the substituent carbon chain.
Alkanes Formula Definition Structure Properties List Of Alkanes Videos Examples And Faqs Of Alkanes
Ch105 Chapter 7 Alkanes And Halogenated Hydrocarbons Chemistry

Question Video Applying The General Formula For Alkane Chemical Formulas Nagwa

Class 10 General Formula Of Alkane Alkene Alkyne Tx Academy Youtube
No comments for "General Formula of Alkane"
Post a Comment